42 Technology Partnership Contributes Insights to WHO Childhood
42 Technology (42T), FIND, the global alliance for diagnostics, and Rutgers University (Rutgers) have collaborated to develop an innovative stool sample processing kit, which has played a pivotal role in informing the recent policy update by the World Health Organization (WHO) aimed at improving the diagnosis of tuberculosis (TB) in children.
In 2020, approximately 1.1 million children worldwide contracted TB out of a total of around 10 million cases. Despite being treatable and preventable, TB diagnosis and treatment in children can pose challenges.
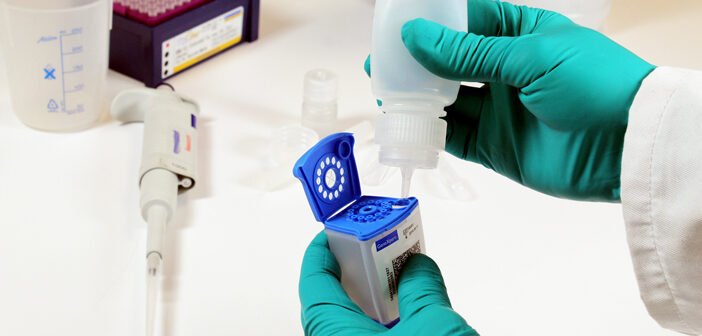
The sample processing kit (SPK) was created to explore whether stool samples, coupled with an automated PCR test, could serve as a viable alternative to traditional smear microscopy and culture techniques for diagnosing TB in children. Over the past three years, multiple research institutions have trialed three different sample processing methods, leading to an analysis of pooled data and the WHO’s recommendation to use stool as the primary sample for TB diagnosis in children up to 10 years old.
“42T, Rutgers, and FIND have successfully collaborated to develop a simple, cost-effective sample preparation kit based on a design and process methodology initially proposed by David Alland, director of the Public Health Research Institute at Rutgers University. Extensive trials have demonstrated the kit’s effectiveness and ease of use in various settings. We are pleased that it has contributed to informing the WHO’s groundbreaking policy update on diagnosing childhood TB,” said Sarah Knight, head of healthcare technology at 42 Technology.
“Diagnosing pediatric TB presents significant challenges and requires innovative approaches because traditional samples for TB testing are often inaccessible from young children. Stool, being easily collectible from almost anyone, represents a major advancement in TB testing. FIND was delighted to collaborate with 42T and other partners to generate crucial data for the WHO review, and we wholeheartedly welcome the guideline update,” said Morten Ruhwald, head of TB at FIND.
TB is typically diagnosed using respiratory samples, which can be invasive and difficult to obtain from children. Stool, on the other hand, is a non-invasive sample but requires extensive pre-processing before analysis using highly sensitive PCR tests.
The user-friendly SPK has been designed to process stool samples effectively without the need for specialized laboratory equipment or technical skills. It is compatible with the Cepheid Xpert MTB/RIF Ultra assay, co-developed by David Alland’s team at Rutgers, FIND, and others, which has been endorsed by the WHO for improved TB diagnosis in both adults and children.
42T’s contributions to the SPK project included working closely with Rutgers to test key device functions, design prototype molded devices, and transfer final designs into mass production to produce initial quantities for use in clinical trials. This effort was supported by a grant from the US National Institute of Allergy and Infectious Diseases.